DNA to Catalyze Your Advanced Therapies
Anjarium Biosciences is a cell and gene therapy tools and technology company headquartered in Switzerland.
Our mission is to enable researchers and manufacturers to accelerate the discovery and delivery cycle of new and evolving genetic medicines. Our technology enables our partners to meet biology’s greatest challenges with increasingly flexible and customizable DNA constructs in pursuit of better human health.
Technology
At Anjarium, we help our customers optimize their R&D and manufacturing workflows by delivering synthetic DNA products with unprecedented speed and customization. We deliver faster, cleaner, infinitely flexible, high-fidelity, hairpin-ended, linear, double-stranded DNA products to accelerate the development and delivery of advanced therapies across diverse modalities.

The benefits of the Anjarium DNA platform include:
Unmatched speed of production and delivery – in a fraction of the time for traditional plasmid
The scale you need – from microgram to multigram quantities
The highest product consistency, stability, and quality
Customizable terminal DNA structures and complex transgene sequences for a variety of applications
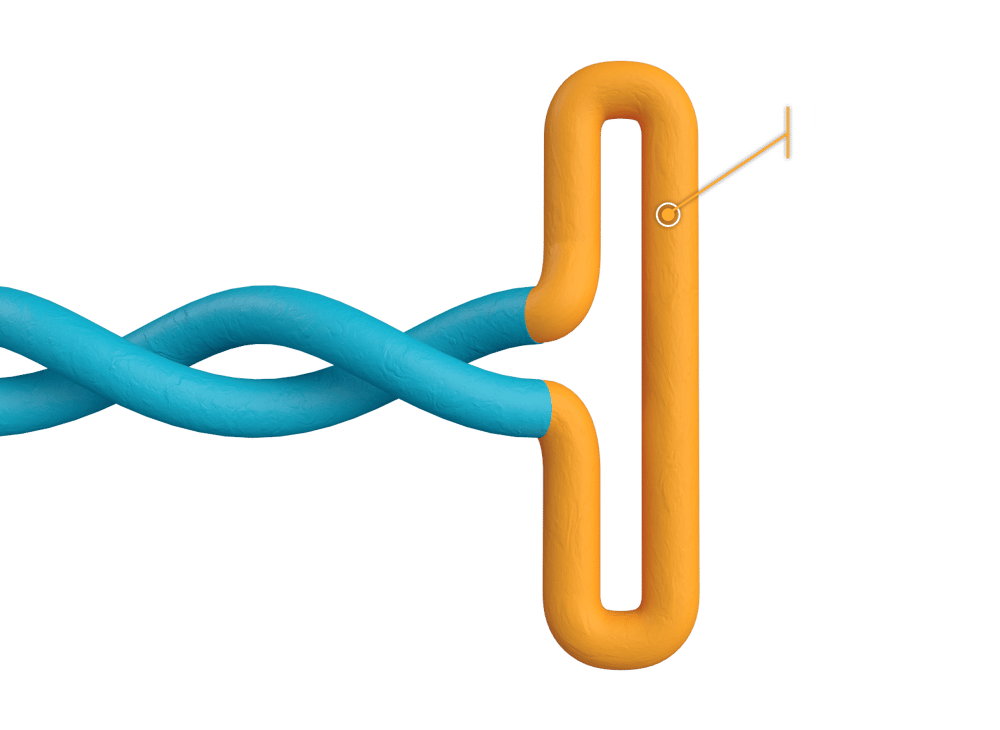
Unique structural integrity unlocks functional applications
We have designed a one-pot reaction that generates linear, double-stranded DNA with customizable hairpin-ended structures. The integrity of the DNA sequence of interest is protected by these structures, which are inspired by nature and play a functional role in certain applications.
Innovative Solutions in Synthetic DNA and Vector Production
Explore Anjarium Biosciences' cutting-edge advancements in synthetic DNA and vector production technologies. Our detailed technical posters highlight the innovative methods and superior performance of our proprietary DNA and Resin products across various applications, including gene therapy, Adeno-associated Virus (rAAV) vector production, mRNA production, and lentiviral vector production and purification. Discover how our solutions are revolutionizing the gene and cell therapy field with higher efficiency, purity, and scalability, driving the next generation of biomedical research and clinical development.
Services
Infinitely flexible, highly scalable synthetic DNA customized to support your needs
We offer a range of products to support research, development, and commercialization of genetic medicines.
Our cell-free platform creates pure, customizable, and flexible synthetic linear DNA constructs for a wide variety of therapeutic modalities – all scaled to your needs.
Why are our products different?
Purity
Unlike with plasmid-based systems, no bacterial sequences are used in production, reducing the risk of unwanted elements and DNA backbone components in end product
Scale
Our process scales from microgram to multigram batches while using a small bioreactor and minimal reagents
Speed
Production time from circular DNA template to vial delivery of weeks rather than months for plasmid-based techniques
Stability
Hairpin-ended structures protect the integrity of the double-stranded DNA sequences
Flexibility
Complex and customized transgene sequences beyond what can be delivered by bacteria-derived DNA
Solutions
What can we do for you?
| Discovery | Preclinical | Clinical | Commercial | |
|---|---|---|---|---|
| RUO |
|
|
||
| GMP |
|
|
GMP |
| Discovery | ||
|---|---|---|
| RUO |
|
|
| GMP | ||
| Preclinical | ||
|---|---|---|
| RUO |
|
|
| GMP |
|
|
| Clinical | ||
|---|---|---|
| RUO | ||
| GMP |
|
|
| Commercial | ||
|---|---|---|
| RUO | ||
| GMP |
|
|
Facilities

Schlieren: Innovation and R&D center
- Analytical development and QC fully equipped
- Executing according to ISO9001 principles

Basel: GMP manufacturing facility
- GMP certification in progress
- RUO, HQ GMP, and GMP
- Swissmedic awarded advanced therapy medical products (ATMP) license for cell therapy production in 2022
DNA offerings
AAV
- ITR stability with significant improvement in yields
- Catalog: Rep/Cap and Helper, Luciferase and GFP reporters in multiple serotypes
- Bespoke: GOI, Helper and Rep/Cap with full serotype capabilities
mRNA
- Less DNA required for equivalent yields
- Catalog: Luciferase and GFP reporters
- Bespoke sequences with stable Poly-A tails up to 140+
Lentivirus
- Production of all constructs including GOI with long terminal repeat (LTR) sequences
HDR and other applications
- Design and manufacturing services to create custom constructs
AAV
- ITR stability with significant improvement in yields
- Catalog: Rep/Cap and Helper, Luciferase and GFP reporters in multiple serotypes
- Bespoke: GOI, Helper and Rep/Cap with full serotype capabilities
mRNA
- Less DNA required for equivalent yields
- Catalog: Luciferase and GFP reporters
- Bespoke sequences with stable Poly-A tails up to 140+
- Production of all constructs including GOI with long terminal repeat (LTR) sequences
HDR and other applications
- Design and manufacturing services to create custom constructs
Contact us about how Anjarium DNA services and products can help your company.
Team
Our team of innovative scientists and manufacturing experts are relentless problem-solvers committed to creating new possibilities for genetic medicines through synthetic, enzymatically produced DNA.
Our Leadership team and Board of Directors bring deep development, manufacturing, and commercialization experience from leading global life science companies.
Investors
Anjarium is proud to partner with leading life science investors.
Investors include Abingworth, Gimv, Omega Funds, Pfizer Ventures, and Surveyor Capital (a Citadel company).


Careers
Join us to accelerate the development and delivery of novel, life-changing medicines to patients.
Anjarium is assembling a world-class global team to accelerate access to revolutionary cell and gene therapies with next generation tools and technology.



News & Events
Contact
How can we help?









